|
Internal Gastric stimulator or gastric pacing |
|
In 2003 when large numbers of gastric bypasses were being done and
consequently, a high incidence of complications were being observed, a
much higher complication rate than formerly thought, the IGS, a pacemaker
like device seemed to hold great hope in the realm of obesity surgery. Pacemakers for the stomach are not new. They have been used for gastroparesis (stomach does not empty) for at least a few years with a modicum of success (apparently not enough to make it the standard treatment, however). And of course we are all familiar with heart pacemakers. The original IGS procedure was invented by Dr Valerio Cigaina, a Italian surgeon and his first patient was his secretary who had about 40 lbs to lose and did it successfully, he claimed, with the IGS. Her smiling face was often seen in the promos for this device. Unfortunately after many unsuccessful trials in the USA, the hope for the IGS had somewhat died. Americans were able to go to Europe to get one but it was expensive ($50,000) and medical follow-up was difficult. What the IGS was supposed to do, is stimulate the nerves of the stomach to prevent or slow the stomach from emptying after eating, causing patients feel "full" for longer and thus eat less. (Since when do we only eat when we are hungry?)
Trouble is, the IGS did NOT work for most people. In the trials the average weight loss was 10-25 lbs, some did not lose any weight at all and only a very few, about 3 patients lost a significant amount of weight (and one of the significant weight losers lost weight by becoming ill with cancer and complications of the IGS). Most of the patients in the trials converted to other procedures to lose their weight.
Like pacemaker patients, IGS patients had to follow certain restrictions with MRIs and scans (i.e. have the pacer turned off before they had any type of radiographic testing done). The pacemaker used in the IGS carried the same risks as the heart pacemaker, some of which are
No one likes to even mention this but of course, anything with a battery runs a small risk of battery related problems i.e. leakage etc. These are very rare but can, I suspect, be nasty when they happen. There were some additional issues with the IGS which do NOT exist with the heart pacemaker. The electrical current generated by the IGS was significantly greater than that of a heart pacemaker i.e. 10 times the intensity or more. In addition to sometimes causing the patient discomfort, (patients complained of numbing or strange feelings in their arms), this also meant that the IGS burned batteries a lot faster than do heart pacemakers. IGS batteries needed to be replaced every 9-12 months - requiring a small surgery.
Surgeons were warned in the instructions for doing the surgery, that the leads must be placed very carefully or else, perforation of the stomach could occur. They also were told to immediately scope for gastric perforation after placing each lead. The companies manufacturing the IGS components tried hard to create a public need by media spin, but the ongoing poor results in the trials caused the media to lose interest in the device. Most of the Bariatric surgeons, involved in the trials, lost interest as well, except for one who took over most of the trials, possibly obtaining by "bait n' switch" many customers for gastric bypass and adjustable lap band.
What the pacer delivered was not what the media promised and of course, the abysmal failure of the American trials was never reported in the media. A couple of years after the promising promos, the Transneuronix website for the pacer disappeared, redirecting visitors to the Medtronix website. A recent visit revealed that there is nothing on the Medtronix site about the pacer (their focus is on many other types of electronic devices including a pacer for gastroparesis. That being said, in 2011, another company popped up with a new "device", a variation of the old IGS. The new company, Abiliti is apparently hawking it for folks whose BMI is 35-55 and suggesting that they can have it removed in a few years when it's done its thing. Longterm repercussions are not mentioned. The battery, so says an ABC news release on it, lasts for 5 years (I bet not near that long) and they of course, don't mention that to replace the battery, requires a surgical procedure. The weight loss results in the studies are the same unimpressive 20%-25% of the excess weight. That means if you have 100 lbs to lose, you might lose 20-25 lbs with the gastric pacer - by far, merely a drop in the bucket. They have reduced the price from the original $50,000 to a slim (?) $24,000.00 but then, one wonders in what way it was cheapened to allow this price reduction. At first viewing, this appears to be the same device Transneuronix sold but a closer look reveals it's much more invasive. The new pacer requires a lead to be placed into the stomach through a hole made in the soft tissue - this detects food coming into the stomach and set off the other lead which turns on the pacer to supposedly make the patient feel full and eat less. The two poster kids are two young men, both not obese and both of whom are physically fit - one of them lost 22 lbs and the other lost 15 lbs with the device. Oddly, the website states both these basically slim men had considered "stomach stapling". huh? The parent company of "Abiliti" is Intrapace, an American firm which according to the news services manufactures heart pace makers. However, if you notice, the intrapace website only talks about the obesity device. According to Linkedin, they were founded in 2001 and are located in Menlo Park, CA. The Linkedin site lists no names of the CEO, etc. To note, even with the old pacer, perforation of the stomach could occur but this new one requires a perforation of the stomach to start with! So you would think with the poor results of the first pacer trials and the fact that the even the company, offers no "poster kids" who were clinically obese to start with and poor weight loss results, the US medical providers would not be interested, right? Wrong - as of 2011, there were 2 trials going on, involving 65 patients none of whom had lost more than 20% of the excess weight (about 20 lbs in a person who is 100 lbs overweight). The British Health Service has approved this device. So much for socialized medicine. :( Articles in peer reviewed journals about the original IGS/GES
|
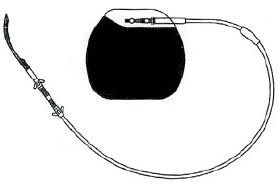 The
great advantage of it was the no "messing" with the digestive system at all
(i.e. no stomach stapling or rearrangment of the bowels or even banding) which
sounded good after more and more gastric bypass horror stories emerged (and even
some not so nice stories about the adjustable lap band which is the safest
obesity surgery available at present).
The
great advantage of it was the no "messing" with the digestive system at all
(i.e. no stomach stapling or rearrangment of the bowels or even banding) which
sounded good after more and more gastric bypass horror stories emerged (and even
some not so nice stories about the adjustable lap band which is the safest
obesity surgery available at present).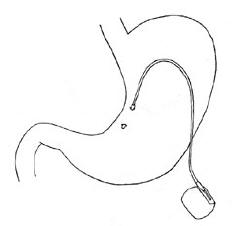 The
poor track record in weight loss was not the only problem with the IGS.
There were several repercussions which made the patients very uncomfortable.
For one, it tended to medically induced GERD, causing patients to
suffer heartburn, and acid reflux (which if happens at night and gets into
the lungs, not only can really HURT but can in the most severe cases, cause a
type of pneumonia). It also raised the risk for Barrett's esophagus, a
pre-cancer condition.
The
poor track record in weight loss was not the only problem with the IGS.
There were several repercussions which made the patients very uncomfortable.
For one, it tended to medically induced GERD, causing patients to
suffer heartburn, and acid reflux (which if happens at night and gets into
the lungs, not only can really HURT but can in the most severe cases, cause a
type of pneumonia). It also raised the risk for Barrett's esophagus, a
pre-cancer condition. Also
patients and providers complained that the IGS was much more difficult to
program than the heart pacemaker (it's programmed with a device which which lies
on top of the skin). If it was programmed to release too little current,
it had no effect on the patient but programming it to release a lot of current
caused discomfort in other areas when the IGS was stimulating the stomach.
The happy medium apparently was never reached in many patients.
Also
patients and providers complained that the IGS was much more difficult to
program than the heart pacemaker (it's programmed with a device which which lies
on top of the skin). If it was programmed to release too little current,
it had no effect on the patient but programming it to release a lot of current
caused discomfort in other areas when the IGS was stimulating the stomach.
The happy medium apparently was never reached in many patients.